-
What is GDPR?
-
What is the role of GDPR in clinical trials?
-
European Union Clinical Trials Regulation
-
Healthcare disaster recovery is essential
-
GDPR clinical trials FAQs
-
Conclusion
Clinical trials contribute to the continuous improvement of diagnostic and treatment procedures and are an indispensable part of medical progress. Closely tied to clinical trials are the laboratory tests and examinations conducted on patients. The process involves handling large amounts of health and genetic data. It is crucial for clinical trials to comply with data protection laws. Since the implementation of the GDPR in European countries, healthcare services and technology providers have comprehensively overhauled their traditional working models to ensure their medical services remain more interconnected and patient-centered.
What is GDPR?
The General Data Protection Regulation (GDPR) is a regulation established by the European Union to protect the privacy and personal data of individuals within the EU and the European Economic Area (EEA). It was enacted on May 25, 2018, with the goal of giving individuals more control over their personal information and simplifying the regulatory environment for international business by unifying data protection laws across Europe. The GDPR imposes strict guidelines on how companies collect, process, store, and transfer personal data.
Under the GDPR, organizations must obtain explicit consent from individuals to process their data, provide the right to access, correct, and delete data, and report any data breaches within 72 hours. Non-compliance with GDPR can result in hefty fines, up to 4% of a company’s global annual turnover or €20 million (whichever is greater). The regulation emphasizes transparency, accountability, and security, aiming to ensure individuals' privacy rights are respected while enabling businesses to operate in a global digital economy.
What is the role of GDPR in clinical trials?
Under the GDPR, health data is considered sensitive data. Facilitating the exchange of health data among member states has become increasingly important in the public health sector, with measures primarily including the provision of healthcare or treatment, prevention of serious cross-border threats to health data, and ensuring high standards of healthcare and the quality and safety of medical products or devices. The GDPR sets out rules for ensuring the legal and trustworthy processing and exchange of health data within the EU, and it also applies to third parties obtaining patient medical data, including patient summaries, electronic prescriptions, and long-term comprehensive electronic health records, as well as the use of this data for scientific research purposes.
Cross-border transfer of clinical trial data involves multiple aspects such as national laws, regulations, and privacy protection. For example, the cross-border transfer of clinical trial data in the United States is protected by the Health Insurance Portability and Accountability Act (HIPAA), which is dedicated to ensuring the security and privacy of medical information. HIPAA requires that personal health information be protected with privacy measures similar to those in the U.S. when transferred abroad. The EU, on the other hand, has implemented the GDPR, which applies to the cross-border transfer of personal data, including clinical trial data. Most non-EU countries also require that data transfers be protected at a level similar to domestic protections.
In the field of DCT (decentralized clinical trials), the GDPR also plays a role. Electronic signatures are increasingly being used in DCT scenarios. Some countries/regions require participant identity verification via video link when providing electronic signatures. Depending on the risk classification and intervention level of the clinical trial, different types of electronic signatures may be required, and all electronic consent forms/electronic signatures must comply with the GDPR. Typically, it is a good practice, and sometimes necessary, to conduct a data protection impact assessment to evaluate risks from a data privacy perspective when using electronic consent forms.
Also, the collected data must adhere to the same GCP standards as any other data collection method. If there are any concerns about data quality, re-monitoring should be considered. Furthermore, all personal data processing within the scope of DCT must comply with the GDPR.
European Union Clinical Trials Regulation
The European Union Clinical Trials Regulation (CTR), is a regulation aimed at standardizing and improving the process of conducting clinical trials across the EU. It was introduced to enhance patient safety, increase transparency, and simplify the approval process for clinical trials, which is particularly important in the context of the rapidly evolving medical research landscape. The regulation came into force on January 31, 2022, replacing the previous Clinical Trials Directive.
The CTR introduces a centralized, EU-wide submission system for clinical trial applications, streamlining the approval process and reducing administrative burdens for sponsors. It also mandates greater transparency, with the results of clinical trials to be publicly accessible through the EU Clinical Trials Register. The regulation ensures stronger protections for trial participants, including stricter informed consent processes and more robust monitoring of trial progress. It also facilitates the conduct of multinational trials, making it easier for companies to conduct research across multiple EU member states.
Healthcare disaster recovery is essential
The healthcare industry's clinical trials are moving toward digitalization and decentralization, with a surge in data volume and deeper global collaboration. Disaster recovery is crucial for clinical trials, as it ensures data security, trial continuity, and compliance with regulations. It helps prevent trial failures or patient risks caused by data loss or system disruptions.
Vinchin Backup & Recovery is designed to meet the data protection needs of healthcare organizations facing digital transformation. With support for multiple virtualization platforms like VMware, Proxmox, XensServer, Oracle, Hyper-V and popular databases, Vinchin ensures high availability and secure data backups. Features such as deduplication, compression, and cloud integration help optimize storage and improve recovery times. Additionally, Vinchin complies with regulations like GDPR, safeguarding sensitive information and ensuring business continuity in case of disasters. This enables healthcare providers to focus on patient care while maintaining reliable data security.
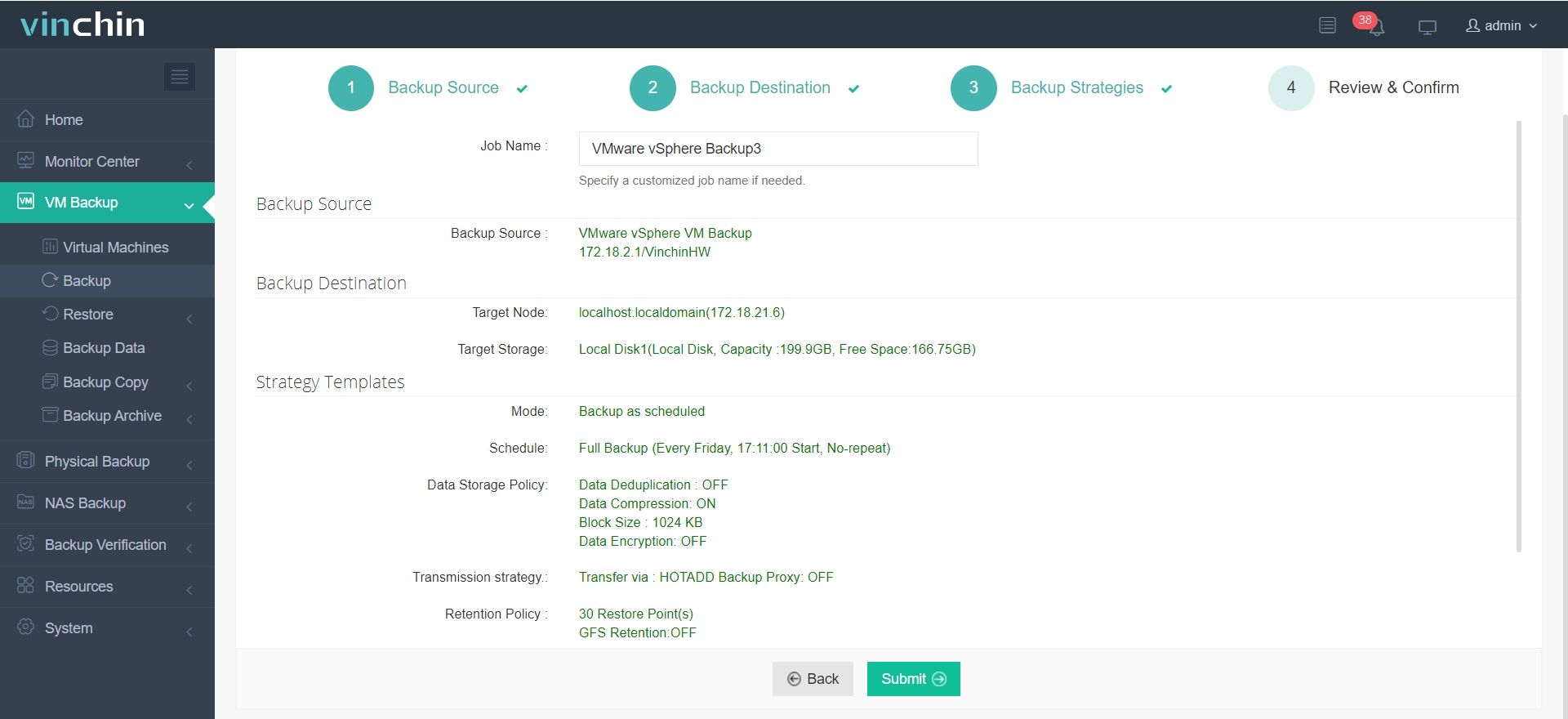
It only takes 4 steps to backup your virtual machine or database with Vinchin Backup & Recovery:
1.Select the backup object.
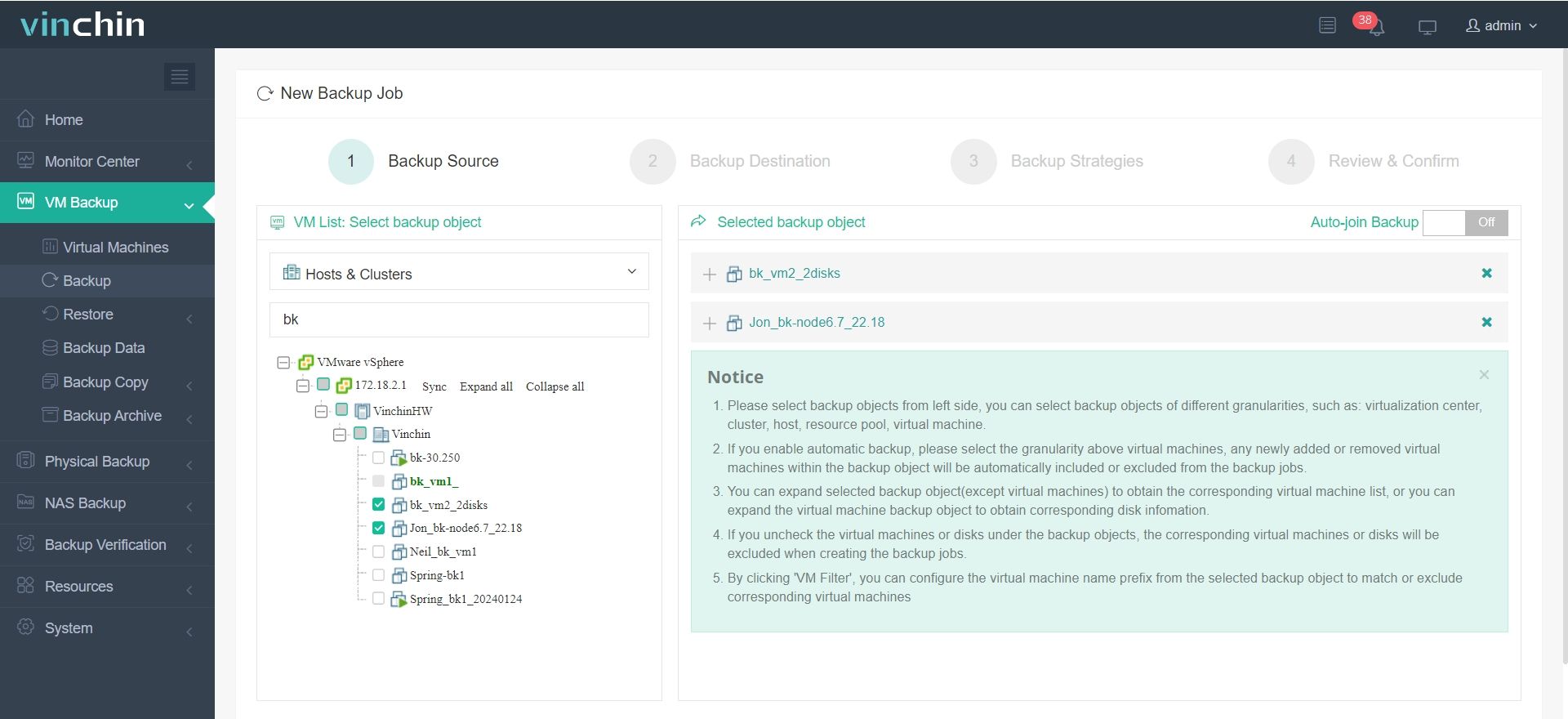
2.Select backup destination.
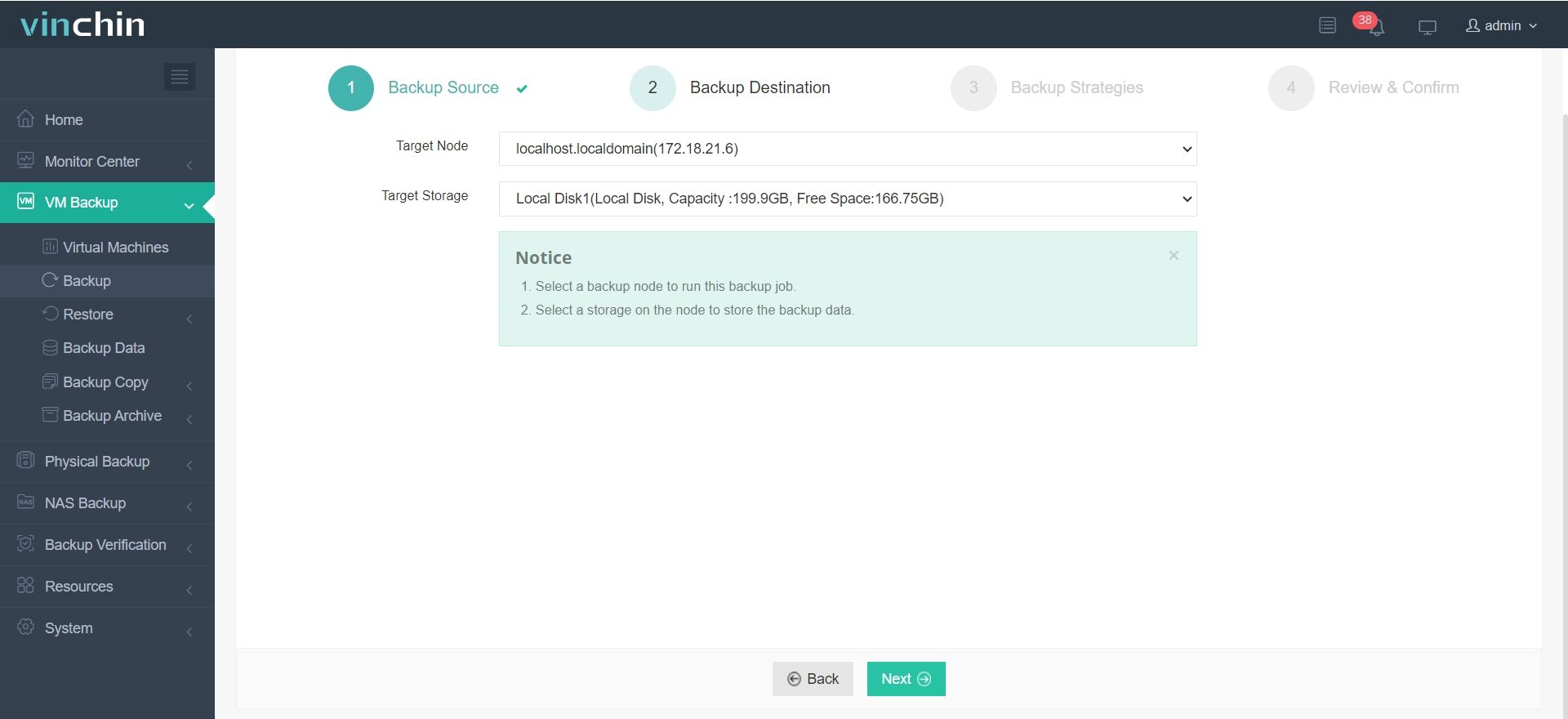
3.Configure backup strategies.
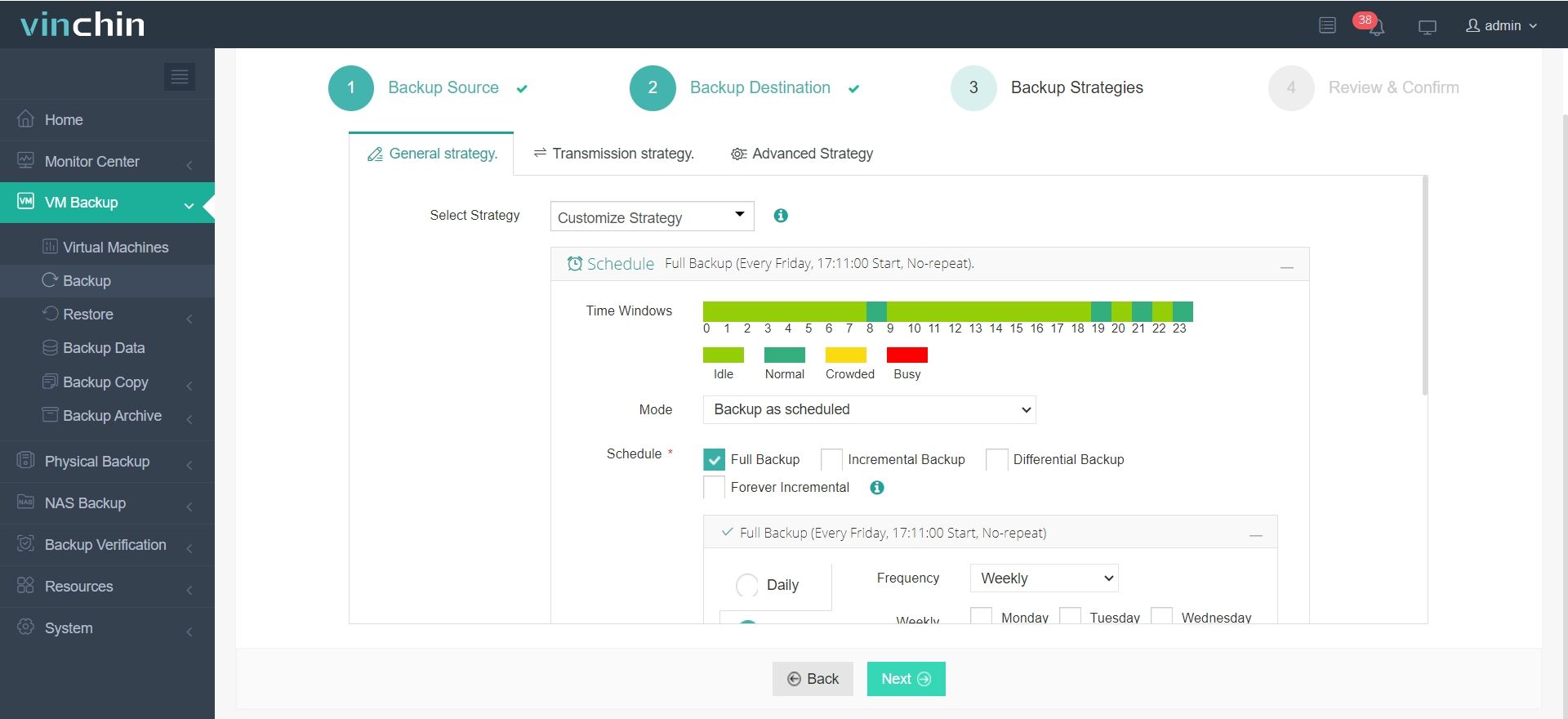
4.Review and submit the job.
Discover the power of this comprehensive system firsthand with a free 60-day trial! Leave your specific needs, and you will get a customized solution that fits your IT environment perfectly.
GDPR clinical trials FAQs
1. What is the difference between GDPR and the CTR?
GDPR focuses on data protection and privacy, while the CTR governs the conduct of clinical trials in the EU. However, the two regulations overlap, and compliance with both is required.
2. Can participants withdraw their consent for data processing after the trial has started?
Yes, participants can withdraw consent for data processing at any time. However, this does not affect the legality of processing that occurred before withdrawal. Sponsors must inform participants of this right during the consent process.
Conclusion
In clinical trials, protecting the privacy of participants is a respect for their fundamental rights and a necessary condition for compliance with the laws and ethical standards of various countries. Researchers and institutions should ensure that all data collection, storage, use, and sharing comply with legal regulations and take effective measures to protect the personal information and privacy security of participants.
Share on: